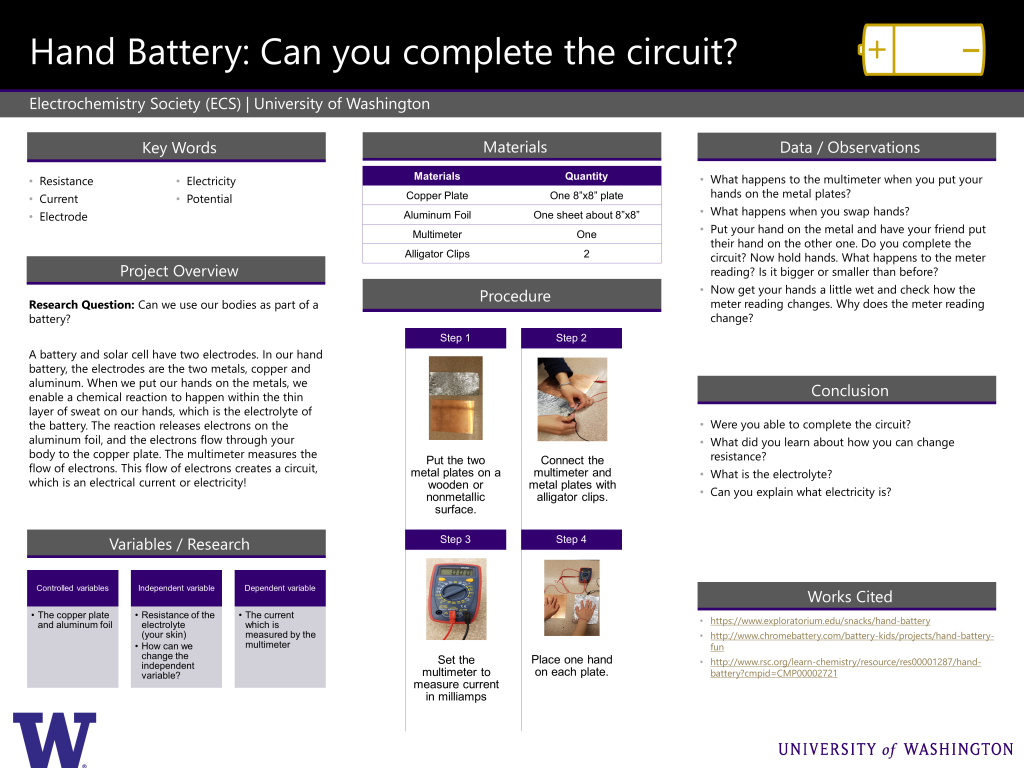
Can we use our bodies as part of a battery?
A battery and solar cell have two electrodes. In our hand battery, the electrodes are the two metals, copper and aluminum. When we put our hands on the metals, we enable a chemical reaction to happen within the thin layer of sweat on our hands, which is the electrolyte of the battery. The reaction releases electrons on the aluminum foil, and the electrons flow through your body to the copper plate. The multimeter measures the flow of electrons. This flow of electrons creates a circuit, which is an electrical current or electricity!
Past Event: Enumclaw STEM Expo “Hand Batteries” in 2018